Author: Sam Angima, Oregon State University Small Farms Program
Publish Date: Fall 2010
As the summer season winds down, it is time again to start planning for next year’s crop. One of the areas to do inventory is soil fertility. Soil pH is one factor that gauges your plant nutrition status. A single soil test may cost over $100, however, soil pH alone may be less costly. Even though the test is inexpensive and can be performed rapidly, many questions are asked about kits, dyes, and portable meters for measuring soil pH.
Many choices for in-field measurement of soil pH are available. The values provided by in-field analyses should be used only as an estimation of soil pH. If an in-field measurement indicates soil pH might be above or below the range given for a crop, send a sample to a laboratory for confirmation.
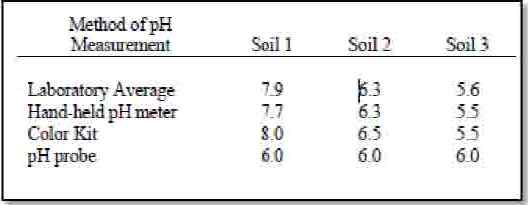
In a national study comparing four in-field soil pH determination methods by University of Arkansas, the hand-held pH meter produced results closest to the average from 82 laboratories while the pH probe provided the same soil pH for three sample groups tested (Table 1). Results from the color kit were intermediate between the pH probe and hand-held meter.The accuracy of the hand-held meters is offset by cost and complicated operation. The hand-held meter is a portable version of a laboratory meter and must have the same care and calibration with buffer solutions before each use. Fresh and accurate buffer solutions are needed for each time the hand-held pH meter is calibrated. The accuracy of the hand-held meter is dependent on the operator’s care and condition of the electrode. A hand-held meter kept in a truck with a dried electrode cannot be expected to perform adequately. Improper storage and treatment of an electrode will not only produce poor results but require replacement before the meter will again perform to standard.
Results from Table 1 show that the soil probe was unable to distinguish between samples with more than a 2.0 pH unit difference, making it unacceptable for use for estimating soil pH in the field. However, the color kit used in this study came with a whole unit (1.0) graduated scale. A smaller graduation pH scale of 0.5 units is necessary in many cropping situations. Sometimes 0.1 – 0.2 unit differences are critical when determining pH given the high cost of lime in Oregon. Solutions used in color kits usually degrade with time and heat. Care is needed to insure the solutions have not degraded. This method can be effective when used to determine which samples will need to be sent to a laboratory for further testing.
Another used method not utilized in this study is pH strip papers impregnated with pH sensitive dyes. Accuracy of pH strips is similar to the color kit and would prove to have nearly similar challenges other than the absence of solutions in pH strips. As with all in-field pH test methods, periodic lab testing is recommended to ensure accuracy with this method.
It is also important to know how the soil lab you use determines soil pH. This is because comparison of results requires same methodology for measuring pH primarily the amount of water mixed with soil. Three common methods for soil pH determination are used by laboratories in the western United States—saturated paste, 1:1 and 1:2 ratio of soil to water. It has been shown that soil pH increases slightly for acidic soils as soil to water ratio increases. Soil pH by saturated paste will be slightly lower than 1:1, which will be slightly lower than 1:2 soil-water ratio. The differences between water measurements will be smaller though at about 0.1 – 0.2 pH units.
Also the time of the year or season when you determine soil pH affect outcome of the results. The highest soil pH is measured in late winter and early spring, prior to fertilizer application. Also pH is high during periods of increased microbial activity and in drier soil. The lowest soil pH (greatest acidity) occurs west of the Cascades near the end of the growing season but prior to the onset of winter rain. In Eastern Oregon the lowest soil pH usually occurs after fertilization in the spring and early summer on irrigated fields. Soil pH can change more than one unit from spring to fall in sandier soils. A common range for seasonal pH change is from 0.3 to 0.5 units higher in spring than during the growing season. The seasonal variation of soil pH west of the Cascades is associated with wetting and drying cycles. Soil pH decreases in the late spring and summer as the salt content of the soil increases.
If you have not determined your soil pH in the last three years, this will be a good time to do so using any method available to you. If you find that the results are not within the range of the crops you would like to grow next year, have the soil tested in the lab early so you can make amendments/additions necessary to ensure better medium for crop growth.